Nanotechnology to the aid of the super-resistant bacterial crisis
Where most modern antimicrobials have failed, nanoparticles might be the alternative we needed to treat infections caused by super-resistant bacterial strains.
by Diego Ramírez Matín del Campo
For eons, microorganisms have engaged in a fierce war in which we have only just begun to become involved. With the emergence of the human race as a dominant species, bacteria's fight over resources and territory moved into new trenches, and their mechanisms of biological warfare became their mean of colonization. Thus, medical research to treat pathogenic bacterial infections became bound to with the development of our societies through the ages, and it was not until the discovery of penicillin by Alexander Fleming in 1928 when we finally got the upper hand; but recent findings have shown that this arms race is long but over, and what once was enough to treat the majority of infections, now finds itself lacking. We enter the post-antibiotic era.
Health scientist preparing foodborne bacteria for a DNA fingerprinting test.
Source: Photo by CDC on Unsplash. https://unsplash.com/photos/tQZ9nTjsQwU
Over the past several decades, bacteria have been gradually developing resistance to drugs usually administrated to treat recalcitrant infections, resulting in the increment of morbidity and mortality in hospitalized groups.
To combat sepsis, multiple or higher doses of antibiotics are needed, leaving no alternative but to use the so-called “last resort” antibiotics in some cases, giving very mixed results. “This type of treatments, besides being expensive, have the possibility of adverse effects and uncertain outcomes.” says a paper published on Natures Reviews Microbiology by J. M. V. Makabenta's team.
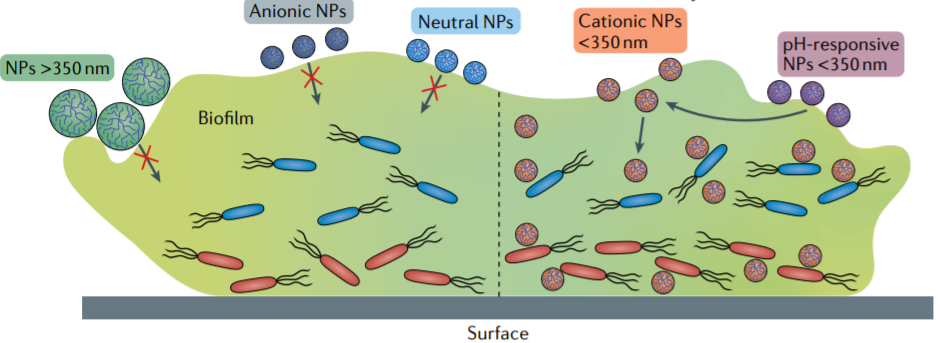
The main issue when dealing with these new bacterial strains is that, not only are they more resistant to the harmful effects of antibiotics due to specific defensive mutations that allow them to endure their attacks, they can also share the genes that grant them these defences with other bacteria, resulting in more super-resistant strains. In addition to that, some species are capable of developing polymeric armour-like structures known as biofilms that, working as barriers protect them from any outside mechanical disruption.
The costs of dealing with this (as stated by the WHO's Antimicrobial Surveillance System) worldwide challenge and international crisis, just like bacterial defences, is getting higher by the minute. Expenses can rise up to US $50,000 per individual, with an estimated US $20 billion societal cost annually in the USA alone.
Here, the excessive use and misuse of antibiotics is to blame. With a plethora of different drugs at our disposal, self-medication and heavy use of pharmaceutical cocktails at minor inconvenience have become routine. The catch here is that not all infections are bacterial in origin, and consuming medicines when not needed can end up acting as a cleansing mechanism that wipes out existing microbiota already present in our system, leaving only resistant bacteria behind. These can then spread and infect an already weakened immune system or share defensive genes resulting in a “bacterial free pass” throughout the body
Source: Photo by Myriam Zilles on Unsplash. https://unsplash.com/photos/KltoLK6Mk-g
“Antibiotic resistance is now one of the greatest threats to human health.” mentioned Pikeville's Medical Center Director of Infectious Disease Physician, Fadi AL Akhrass. “It is important to educate our patients and their families on the importance of the appropriate use of antibiotics.” he continued.
With bacteria regaining the advantages that we once got over them, it almost feels like we are moving backwards; back when something as common as the flu, a “strep throat or a child's scratched knee could once again kill.” said Margaret Chan, former director-general of the WHO at a speech directed to the EU union panel. But in reality, we are not moving back into a pre-antibiotic era. “No. This will be a post-antibiotic era.” continued Margaret Chan. “In terms of new replacement antibiotics, the pipeline is virtually dry, [...] The cupboard is nearly bare. [...] A post-antibiotic era means, in effect, an end to modern medicine as we know it.”
As of right now, getting a bacterial infection doesn't translate to the need for immediate medical intervention. But what is worrying here is the rapid growth at which these types of cases are being presented around the world, being particularly damaging to already hospitalized patients, especially those in intensive care units, surgery, transplantations, and cancer treatment.
In this bacterial arms race we are working against time, and empiricism tells us that there are two main ways in which we can confront this challenge. During the pre-antibiotic days, we used to steal nature's already existing defensive tactics, as we did with fungi's penicillin and with plant´s antimicrobials. Later on, during the antibiotic era, the development and manufacturing of synthetic drugs was the norm. Now we visit both perspectives to see what they offer.
Today, when looking at nature's solutions, scientists are turning to bacteriophages (bacteria's natural enemies) meanwhile on the engineering side, nanomaterials present great promise for therapeutics. Focusing on the latter, nanomaterial-based antimicrobials offer most of the benefits of traditional nature-based drugs with almost no down side. But, what exactly are these almost science-fiction sounding alternatives to traditional drugs?
The word nano come from the greek νάνος (nanos), meaning dwarf; a perfectly suitable name since this structures size the billionth of a meter. The practical dimensions for medical purposes, go from 500nm to 100nm or even less. They can be made out of organic, inorganic, or hybrid materials; their size being their key characteristic.
Bacteria covered by a biofilm, compared against different nanoelements and their sizes.
Source: Photo found in the scientific paper: Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections.
Due to their flexible composition, the structures into which they can be molded are almost unlimited, which is a strong reason why they are so attractive for modern medicine. Varying from wire-shaped structures to rod-like elements, or fibrous networks, to spheres with hollow or solid interiors and smooth or rough surfaces, nanoparticles are only limited by their structure and construction, and it is precisely on the exploitation of these characteristics where we can find the upper hand when dealing with super-resistant bacteria. For example, angular-shaped elements can be used to inflict direct cell wall damage, meanwhile hollow micelles can be used as a cargo delivery system to hand over therapeutics in a specific manner.
Nano-sized elements “act under modalities that are novel to bacteria, and thus are not in their natural defensive arsenal.” said Makabenta's team. This means that nanomaterial-based bactericides are less prone to be selected for resistance than conventional antibiotics due to their not-so-natural way of action.
Let us not forget that nanometric antimicrobials are still the new players on the block, and complementary studies are yet to be implemented. Immediate safety and long-term effects of nanotherapeutics are among the biggest roadblocks before moving into major commercial clinical use. “Current studies are determining the pharmacokinetic profile of nanoparticles to better understand their fate in the body.” said Makabenta's team.
There is an immense variety of components with which nanomaterial-based therapeutics can constructed. Pure element ones are made of elemental carbon or metals. Others mimic cell-like structures, like liposomes or polymeric molecules. And others are dependent on the way their elements mix (if they do) such as nanoemulsions and nanocomposites.
Size comparison between a nanoemulsion, metal nanoparticle, liposome and a bacteria. Source: Photo found in the scientific paper: Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections.
Being human-made, these microelements can be arranged and engineered in such a way that allows us to take advantage of their most beneficial individual qualities, and through planning and design, make them the perfect medicine. For instance, if we take the antimicrobial properties of pure silver and use it to protect a liposomal carrier, it should be able to safely deliver a potent bactericide. We call these structures “smart nanomaterials” and can be designed directly to respond to internal and external stimuli, such as pH and bacterial residues on the inside, and light, temperature, or electromagnetic fields from the outside.
As shown with smart nanomaterials, more often than not, nanoparticles work better in tandem, taking advantage of the various characteristics from individual components. For example, levofloxacin, a broad-spectrum antibiotic, loaded into a silver core-embedded nanocomposite mixture of silica particles showed great antimicrobial effects against multidrug-resistant E. coli strains, and when applied in an in vivo mouse with induced peritonitis, the bacterial burden was then reduced by three orders of magnitude, with no toxicity observed. In another study, carvacrol oil, an essential oil found in oregano and thyme with poor biofilm penetration and potent antimicrobial properties was introduced in an oil-in-water-based polymeric nanocomposite. This allowed the particles to successfully permeate invasive multidrug-resistant biofilms of Gram-negative and Gram-positive bacteria while also maintaining minimal cytotoxicity towards mammalian cells.
Although promising, we are in nanotechnology-derived therapeutics early steps. Extensive tests and trials tell us that they work, yet the exact mechanisms of actions of some of these nano-bactericides are yet to be understood. That is the case, for instance, of silver-based and some of carbon-based elements. Scientists believe that their bactericidal effects come from direct mechanical disruption, lethal oxidative stress, also known as ROS, and specific binding to intracellular components, but the exact mechanisms remain unknown. Nevertheless, results are promising and investigation keeps on smooth sailing. Currently, nanoparticles-based therapeutics have been applied successfully on ex vivo and in vitro models, as well as on in vivo rat models with minimal cell damage and next to no toxicity levels.
Nanomaterial's distinct mechanisms of action. Source: Photo found in the scientific paper: Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections.
In addition to their direct mechanisms of action, their range is not limited to the direct killing of bacteria. For instance, magnetite nanoparticles locally heated through the exposure of an alternating current magnetic field, resulted in the dispersion of bacterial cells. Some nanoelements can also be used to block communication between colonies. Silicon dioxide nanoparticles covered with β-cyclodextrin successfully stopped Vibrio fischeri cell's communication, which then reduced their proliferation. As these mechanisms of action do not include the direct killing of bacteria, the development of long-term resistances appears to be highly improbable.
Studies keep on moving forward at a steady pace. In 2019 at least 30 nanoparticle delivery systems were in phase I clinical trials, and arround the same numbers were in phase II. By 2020, two liposomal nanoformulations for controlled delivery of antibiotics were in phase III clinical trials. At the same time, new guidelines are being developed for appropriate in vitro and in vivo models exploration depending on the type of infection. “Systemic safety and long-term effects of nanoparticles on the body are still among the major barriers to clinical use.” cites Makabenta's paper. “Current studies are determining the pharmacokinetic profile of nanoparticles to better understand their fate in the body.”, it continued.
And so, technological innovation emerges from the need and the collective efforts of different branches of human knowledge, proving the path ahead as an interdisciplinary one. “Interdisciplinary collaborations among chemists, biomedical researchers, including microbiologists and engineers [is required]. Likewise, the partnership between fundamental translational and industrial agencies will be instrumental in moving antimicrobials to the clinic.” stated the researchers over at Natures Reviews Microbiology study.
It is not the first time we've found ourselves stuck between a rock and a hard place, scientifically and medically speaking. The long-lasting, still present pandemic crisis is a living testimony. History tells us that this won't be the last time either, but pertinent investigation, adequate funding, and the interdisciplinary efforts of different practices and branches of human knowledge and technology prove that we are heading in the right direction. We enter the post-antibiotic era in full throttle, but also into the age of nanomaterial-based therapeutics.