Your Practical Guide for Protein Brewing
Your Practical Guide for Protein Brewing
By Chiara Lonigro, PhD student at VIB-UGent, Belgium
You are probably wondering why should you ever want to brew proteins? Well, proteins are everywhere! They are fundamental components of all living organism: your hair, your skin and your nails are all made of a special protein called keratin. Proteins are essential in our diet and we consume them with nearly every food we eat.
A beautiful star-shaped protein: crystal structure of the serine proteinase inhibitor ci-2 from barley seeds (2CI2), Image source https://www.rcsb.org/structure/2ci2
Enzymes are the proteins at the base of several industrial applications such as biological detergents, lactose-free dairy and fruit juice manufacturing processes.
Biologics are pharmaceuticals that are made by living organisms and they are protein based-drugs, whether you are a diabetic patient taking insulin or took a hepatitis B vaccine you are using human-made proteins.
Proteins are also beautiful, look at the chymotrypsin inhibitor that’s evolved into the shape of a star! Because proteins are so interesting and useful, scientists learnt how to produce them in the lab and use them for different purposes.
Making proteins is quite difficult, but living organisms build them every day to grow and survive. Take microbes for example! These are living organisms, but tiny (micro!): did you know that one tablespoon of a microbial liquid culture can contain up to 800 billion bacteria? Everyone also has experienced how fast microorganisms can grow! Yes, like those on the cheese that was only a few days old… No need for lot of space, fast, easy to grow, so why not to use microorganisms to make proteins? That’s indeed what you are going to learn today! Excited? Let’s start!
1. Choose your favourite protein
Whether you like it big, small, colourful, or not, proteins are all made by the same building blocks called amino acids. A DNA sequence specifically codes for an amino acid sequence, thus for a protein. But you can work backwards too. You can easily translate your protein’s amino acid sequence into a DNA sequence
Image credits: Chiara Lonigro
by matching up the amino acid building blocks to the DNA code, scientists call it Reverse Translation. Once you know your DNA sequence, you can copy and paste it into your favourite microorganism. Can’t decide? Check https://www.rcsb.org/, the largest protein database where you can find all the proteins with known protein structure and many more information.
2. Pick your favoured microorganism
It is true, there are plenty of microorganisms out there, however you better choose wisely. Scientists know some microorganisms reeeeeally well! They know every single piece of DNA that makes their genome and they learnt how to modify it. The bacterium Escherichia coli and the yeast Saccharomyces cerevisiae (a.k.a. baker’s yeast) are usually researchers’ favourites, at the point that they called them ‘model organisms’. Fancy, isn’t it?
Very Important Microbes (VIM): the model organisms Escherichia coli and Saccharomyces cerevisae, Image source: https://www.deviantart.com/velica/art/Model-Organisms-E-coli-133396423
https://www.deviantart.com/velica/art/Model-Organisms-Yeast-133460328
Whatever organism you pick, it will read the DNA sequence we have inserted into the organism in the same way and connect the amino acids to make your protein. However, a protein is not a mere sequence of its amino acids, the amino acid string also needs to fold up into its proper structure (remember the star-shaped chymotrypsin inhibitor)?
Some proteins require special modifications, for example our antibodies (which are proteins) need extra sugars attached to their surface to work. Insulin requires a modification called a disulfide bond, which keeps different protein pieces bound together tightly. These same bonds decide whether your hair (also made by proteins!) will be straight or curly.
Different organisms are equipped with different instruments to help the protein to assume its proper form and modifications, only if you picked the right one you will get your protein. For example, the bacterium E. coli is not equipped to make these sugars and disulfide bonds. You could choose to make a ‘perm’ to your ‘straight’ bacterial-produced insulin afterwards using some chemical reagents, or let a yeast produce the protein in the first place, which is better equipped for making these ‘curly’ proteins. '
3. Make your genetically modified microorganism
Once you have chosen your microorganism, you can insert the piece of DNA that codes for your favourite protein into its cell. There are several ways to do that, for example, you can ‘shock’ your microbes with heat or magnetic field, to create tiny holes on the cell surface from which the DNA can enter.
Once inside, the cells will believe that the piece of DNA is their own and they will start to read it to produce your protein. An organism that has been modified in such a way is called “Genetically Modified Organism”, better known as GMO.
GFP in the making: plate full of genetically modified bacteria (E. coli), Photo credits: Enrico Ferrarini
4. Let your tiny friend grow and brew your protein
Bacteria and yeast replicate very quickly and most of the time they don’t even need a partner! The bacteria E. coli duplication time is approximately 30 minutes. Ideally, if you start with only one bacterium, after 12 hours you will have more than 16 million of its copies, each containing your DNA and producing your protein.
If you decided to pick the yeast S. cerevisiae, you will have to wait a bit more time to multiply this chubby microbe, since it takes about 2 hours to duplicate, however, one cell can usually produce more protein than a bacterial cell.
All this happens in a special place called fermenter or bioreactor. The bioreactor is a 5-star hotel for microorganisms! Every parameter is controlled to allow for the best conditions to make the microbes happy and with enough food. The happier the microbes, the more they’ll grow and produce your protein!
5. Find your protein and purify it from the broth
You’re almost there, your protein has been produced! Can you see it? I guess not! Most proteins can’t be seen with naked eye because they are too small. Insulin is 5000 times smaller than the width of a human hair! In fact, hair is made up of millions of proteins rolled up together. Yet, you can see my favourite protein because it’s green! It is the green fluorescent protein of the jellyfish Aequorea Victoria, a.k.a. GFP for its friends.
GFP in the making: broth of GFP producing bacteria (E. coli), Photo credits: Chiara Lonigro
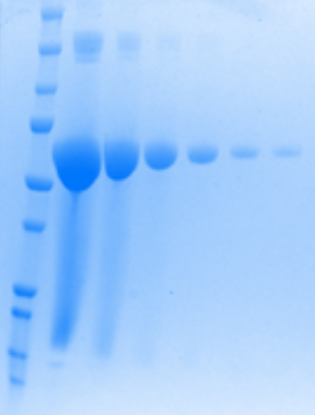
Even though the majority of proteins are colourless, scientists found ways to indirectly see them. For example, there are some coloured dyes that we can use to stain proteins. Can you see the blue bands in this image? Guess what, yes, it’s your protein!
GFP in the making: GFP stained with coomassie blue dye, Photo credits: Chiara Lonigro
Your protein is there, now you can see it! However, it’s in a mix of cells, other proteins, and microbial nutrients that you want to remove. Especially if you are producing a drug, such as insulin, you need to make sure that your protein is 100% pure. You can do that in different steps.
The first is to get rid of the microbes. Because cells are bigger and heavier than your protein, you can use a filter or a centrifuge.
GFP in the making: Machine used to purify proteins from the broth at laboratory scale, Photo credits: Enrico Ferrarini
In a second step, all the other molecules have to go, you just have to find what is special about your protein and use that property to separate it from the rest. Every protein has a specific size, structure, and charge, which determine with what your protein is going to interact. Insulin is slightly positively charged, and you can use interactions with a negatively charged polymer to selectively capture your protein, and there you are! Congratulations, you made it! You became a protein brew master!
Easy peasy isn’t it? In fact, the production of proteins in microbial factories is a field in continuous expansion although there are still many technical limitations that we have to overcome. There is an unimaginable microbial diversity out there that is yet to be discovered and from which we can learn. Protein production is only one of the incalculable reasons why microbes are so important in shaping our world! Follow International Microorganism Day on September 17 to discover more!